Likelyhoid of Hemorrhage After Birth Will Happen Again
Postpartum bleeding occurrence and recurrence: a population-based written report
Med J Aust 2007; 187 (7): 391-393. || doi: 10.5694/j.1326-5377.2007.tb01308.x
Published online: i Oct 2007
Postpartum haemorrhage (PPH) — excessive haemorrhage afterwards childbirth — is one of the leading causes of maternal mortality in the adult world.1 The incidence of PPH is increasing.2-four PPH may arise de novo in whatever pregnancy, and may recur in a subsequent pregnancy. Previous studies have reported an increased hazard of PPH with a previous occurrence, but these studies relied on self-report of previous PPH status,4,5 were infirmary-based and not population-based,5,six involved pocket-size numbers of women,7 excluded caesarean deliveries,five-seven and ignored parity.5 The probability that maternal weather condition similar PPH will occur or recur informs clinical determination making, maternity service provision and guideline evolution.8
Given Australia'due south geography and population distribution, ensuring that women give nascency in hospitals that have the facilities to care for them and their babies relies on a organisation of regionalised intendance. The power to place antenatal risk, assuasive transfer of mothers before they requite birth, is key to successful regionalised maternity care. Transfer of mothers to advisable facilities earlier they give nativity has been shown to lower rates of perinatal mortality and morbidity and reduce periods of hospitalisation compared with postnatal–neonatal transfers.nine,10 Quantifying the probability of an adverse issue will identify mothers who are likely to need increased care and monitoring, thus minimising or avoiding future maternal and perinatal morbidity and bloodshed.
Here, we study the risk of occurrence and recurrence of PPH in subsequent pregnancies as adamant using tape linkage of women's singleton pregnancies over fourth dimension.
Methods
Data relating to consecutive singleton pregnancies and PPH were obtained from population-based linked nativity and infirmary-discharge records that were probabilistically linked and de-identified by the New Due south Wales Department of Wellness using previously described methods.11,12
These data were used to identify women having at least a first and second singleton pregnancy resulting in a birth at > 400g or ≥ 20 weeks' gestation in the menstruation ane January 1994 to 31 December 2002. Women with a first delivery before 1994, with pregnancies that were not consecutive, or with parity information missing for whatever pregnancy were excluded.
PPH was diagnosed during the birth admission by attention obstetricians, clinicians or midwives according to the Australian version of the International nomenclature of diseases guidelines (500 mL or more of blood loss later vaginal delivery or 750 mL or more after a caesarean delivery13,14). Validation of PPH recording against medical records has shown that PPH rates are under-enumerated (sensitivity 58.6%), but with high specificity (99.viii%)xv indicating few fake-positive reports. We did not include blood transfusions as a marker of severity in our primary definition and analysis of PPH because of a dramatic linear (squared correlation coefficient [R2], 0.92) increase in the year-by-year transfusion rate amidst women with PPH during the written report period (2% in 1994 to 12% in 2002).ii Consequently, the transfusion charge per unit was higher in the start and subsequent pregnancies that occurred later in the study period. However, we undertook a secondary analysis to run into if the patterns of occurrence and recurrence were similar when a more stringent definition of PPH (PPH with transfusion) was used while accounting for this groundwork increase in transfusion rates.
Mode of delivery was identified from the birth data: caesarean deliveries included those with and without labour; vaginal deliveries included instrumental vaginal deliveries.
Statistical analysis
We determined the gamble of the first occurrence of PPH in first, second or third pregnancies, and the risk of recurrence for women with a history of PPH in their first and/or 2nd pregnancies using contingency tabular array analysis. Adventure ratios (RRs) and 95% confidence intervals were calculated for the recurrence risk in second and third pregnancies compared with women with no history of PPH in the equivalent pregnancy.
The study was approved by the Academy of Sydney Human Enquiry Ethics Committee.
Results
Afterwards excluding 45 640 women with a first delivery earlier 1994, eleven 572 with pregnancies that were non consecutive and 442 with parity information missing for whatever pregnancy, information for 125 295 women having at to the lowest degree a outset and 2d singleton pregnancy resulting in a birth at > 400g or ≥ xx weeks' gestation in the study period were available for analysis. Of these women, 23 095 had at least 3 consecutive singleton pregnancies.
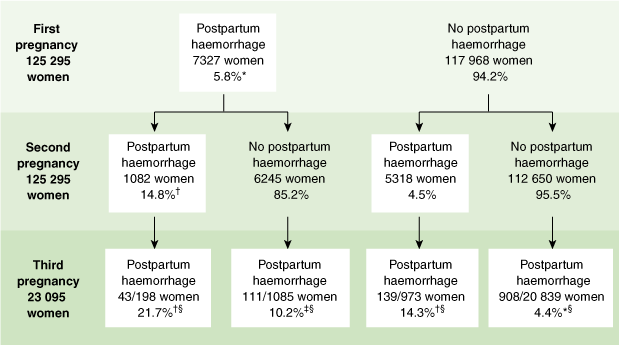
Among the 125 295 women with consecutive pregnancies, 5.8% had a PPH in their first pregnancy, and, of these, xiv.8% went on to accept a recurrent PPH in their second pregnancy (Box). This represents a risk of recurrent PPH iii.iii times higher than for women with no history of PPH (RR, iii.three; 95% CI, 3.1–three.5). The recurrence chance rose to 21.7% for women who had had two prior PPHs compared with four.4% for those with no history of PPH (RR, 5.0; 95% CI, iii.8–six.5). Findings were similar when the first PPH occurred in the second pregnancy (4.v%) and then the subsequent pregnancy (14.iii%) (Box). In women who had an intervening pregnancy without a PPH (ie, a PPH in a showtime and 3rd pregnancy only) the risk of PPH recurrence in the tertiary pregnancy was ten.2% (Box).
A similar pattern of occurrence and recurrence was found when PPH was redefined equally requiring transfusion, and prior-year transfusion rates were trend-adjusted to match the 2002 base transfusion rates — 0.vii% PPH in the first pregnancy; 0.5% first PPH occurrence in the second pregnancy and 5.9% recurrent PPH in the 2d pregnancy (RR, 11.4; 95% CI, vii.9–16.five). Two women who had a PPH requiring transfusion in both their offset and second pregnancies had tertiary pregnancies, and one had a third PPH requiring a transfusion.
The increased risk of recurrence was also axiomatic when mode of delivery was taken into account. For women with consecutive vaginal births, risks were: 6.ii% PPH in the commencement pregnancy; 4.8% first PPH occurrence in the second pregnancy; 16.five% recurrent PPH in the second pregnancy (RR, 2.7; 95% CI, 2.5–two.8); 4.3% offset PPH occurrence in the tertiary pregnancy; and 22.5% for a third PPH (RR, 3.half dozen; 95% CI, 2.7–4.eight). For women with consecutive caesarean births, risks were: 3.2% PPH in the first pregnancy; 1.9% showtime PPH occurrence in the 2nd pregnancy; and 4.9% recurrent PPH in the second pregnancy (RR, ane.v; 95% CI, 1.1–2.two). Vi women who had PPHs at their first 2 caesarean births had third caesarean births, and two had a PPH.
Discussion
Our study shows a remarkable consistency in the chance of first PPH occurrence, regardless of parity, and an increased hazard of PPH recurrence. We found that the risk of a start PPH in any pregnancy is ane in xx (take a chance of about 5%), while the chance of recurrent PPHs increases to one in seven for a 2nd pregnancy and one in 5 for a third. The risk of a recurrent PPH in subsequent pregnancies is substantially elevated even when an intervening pregnancy is uneventful. Although the risk of outset PPH requiring transfusion was much lower (<1%), information technology was over again consistent past parity and the recurrence risk was very high. Women who had a showtime PPH requiring transfusion were 11 times more likely to have a recurrent PPH with transfusion than women with no prior history of PPH requiring transfusion.
The strengths of our study include the use of longitudinal population-based data, giving us admission to data on rare outcomes16 and the employ of validated obstetric items for which accuracy and reliability of reporting have been quantified.xv,17 Weaknesses of our study include the inability to assess the severity of PPH and to differentiate between whether the third stage of labour was managed actively (using a prophylactic oxytocic medication before delivery of the placenta, early on cord clamping and cutting, controlled umbilical cord traction) or expectantly (allowing the placenta to deliver spontaneously or aided by gravity or nipple stimulation).18 While it is possible that recording of recurrent PPHs is subject to recording bias (having been previously identified as having a PPH may increase the likelihood of further recording), this is unlikely to explain such a large increase in risk.
The recurrence hazard we report (RR, 3.3; CI, three.one–3.5) is very similar to that found in the few previous studies that have been undertaken. The three other studies plant that having a history of PPH increased the hazard of a recurrence of PPH in a subsequent pregnancy past between 2.2 and 3.3 times compared with women with no history of PPH.5-7 The simply other report to investigate recurrence in a third pregnancy plant that 25% of women (compared with 22% in our study) with two prior sequent pregnancies with a PPH went on to have a 3rd PPH.7 It should be noted that all previous studies limited analyses to women with consecutive vaginal deliveries, primarily because quantifying the take a chance of PPH recurrence was not the master focus of the study.5-7
We wish to stress that direct comparison of PPH run a risk past obstetric interventions, such equally induction or augmentation of labour or style of delivery, is problematic. Such interventions are inextricably linked to other risk factors for PPH including maternal medical weather condition, placental abnormalities, prolonged labour, genital tract trauma and fetal size. For case, placental abnormalities are associated with PPH risk but are also an indication for caesarean delivery.19 Neither the reasons for interventions, nor the temporal sequence of events tin exist determined from population health datasets. Therefore, attributing risk to an intervention could exist misleading. Consequently, our data on PPH recurrence by mode of commitment should exist interpreted with circumspection. Although the absolute take chances of PPH was lower for women having caesarean sections, this may be related to the higher blood loss needed to fulfil the definition of PPH after a caesarean section,thirteen,xiv the difficulty estimating blood loss at caesarean section,20 and differential nether-reporting in population health datasets by mode of delivery (sensitivity of PPH reporting for vaginal births, 77%; and caesarean sections, 43%).21 Furthermore, stratifying results by style of delivery results in very small numbers of women with recurrent events, particularly among third pregnancies. We do not believe our written report provides sufficient item on timing and indications for delivery to make recommendations nigh future style of commitment. Still, chiefly, regardless of the mode of delivery there was an increased risk of recurrent PPH.
Longitudinally linked population health data present a unique opportunity to study recurrence risks — using large sample sizes with consistent reporting over time facilitating generalisable results.22 Our cohort could be extended as more information become bachelor, thus increasing the number of women with consecutive births and improving the precision of our recurrence estimates. Furthermore, linkage of birth records to subsequent hospital admissions would enable ascertainment of boosted secondary PPHs (occurring between 24 hours and 12 weeks subsequently nativity).
As PPH is a major cause of maternal morbidity and mortality,23,24 and the risk of recurrent PPH is high, we suggest that women with a PPH in a prior pregnancy should be delivered in hospitals with onsite claret cross-match facilities. In NSW, only 4% of hospitals providing maternity care do not take cross-match facilities (all are small-scale rural hospitals) and, in making this recommendation, nosotros summate that just 178 (2.ane/1000) of the 85 000 women giving birth in NSW would be affected past a policy recommending commitment in a hospital with an onsite blood-banking concern for women with a prior PPH.
Reporting the risks of recurrent PPH enables informed risk-counselling of pregnant women virtually the well-nigh appropriate place to requite nascence and the need for rigorous awarding of agile management of the third stage of labour.xviii If i in seven women will have a 2d PPH and one in five women who have had a second will take a third PPH, consideration should be given to subsequent delivery in a infirmary that has onsite cross-lucifer facilities.
Occurrence and recurrence of postpartum haemorrhage among consecutive singleton pregnancies, New South Wales, 1994–2002
| | |||||||||||||||
| | |||||||||||||||
| Note: All first pregnancy records accept a second pregnancy record, just non all 2d pregnancy records have a tertiary pregnancy recorded. * Occurrence; † Recurrence; ‡ Recurrence with an intervening uneventful pregnancy. § Proportions are calculated based on women who went on to have a third pregnancy. | |||||||||||||||
Received 13 Feb 2007, accustomed sixteen July 2007
Source: https://www.mja.com.au/journal/2007/187/7/postpartum-haemorrhage-occurrence-and-recurrence-population-based-study